Edward Mills came to the meeting last month with very good data. A clinical trials expert at McMaster University, Mills was presenting new results from a trial that is looking at how well half a dozen different drugs treat Covid-19—not for the people so sick they’re in the emergency room or the hospital, but in people whose symptoms haven’t gotten that bad yet. People sick at home, in other words.
At his online talk, put on by the National Institutes of Health, Mills’ slides told the tale: A relatively safe, familiar, cheap drug reduced the relative risk of mild Covid getting worse by nearly 30 percent. The drug is fluvoxamine, a selective serotonin reuptake inhibitor—an antidepressant. (It’s also an anti-inflammatory, and inflammation and an overreacting immune system are hallmarks of serious Covid infection, so that might be why it seems to help). Get a bunch of people with Covid and randomize them into two groups; 739 get fluvoxamine and 733 get a placebo. Only 77 of the fluvoxamine-takers end up in the hospital; 109 of the placebo group do. This is exciting.
“This is the first time these results have been presented in a public forum?” asked the moderator, Adrian Hernandez, director of the Duke Clinical Research Institute.
“Yeah,” Mills answered. “You are hearing it for the first time.”
“Well, simply, wow,” Hernandez said. If the data bears out, it’ll be only the second repurposed drug that works for outpatient Covid-19. (The other is a steroid called budesonide; other drugs you might have heard of, like remdesivir or dexamethasone, are for people who are severely ill and hospitalized.) The team’s results haven’t been peer-reviewed or officially published yet, but the Together trial, on which Mills is co-principal investigator, is well-designed and respected. Now, to be clear, fluvoxamine is still a ways off from becoming part of the standard of care for people with Covid-19. Once the Together trial’s results get published, guideline-setting organizations like the US Food and Drug Administration and the World Health Organization will have to take a look. But the Together trial data, if it holds up, seems positive for the SSRI.
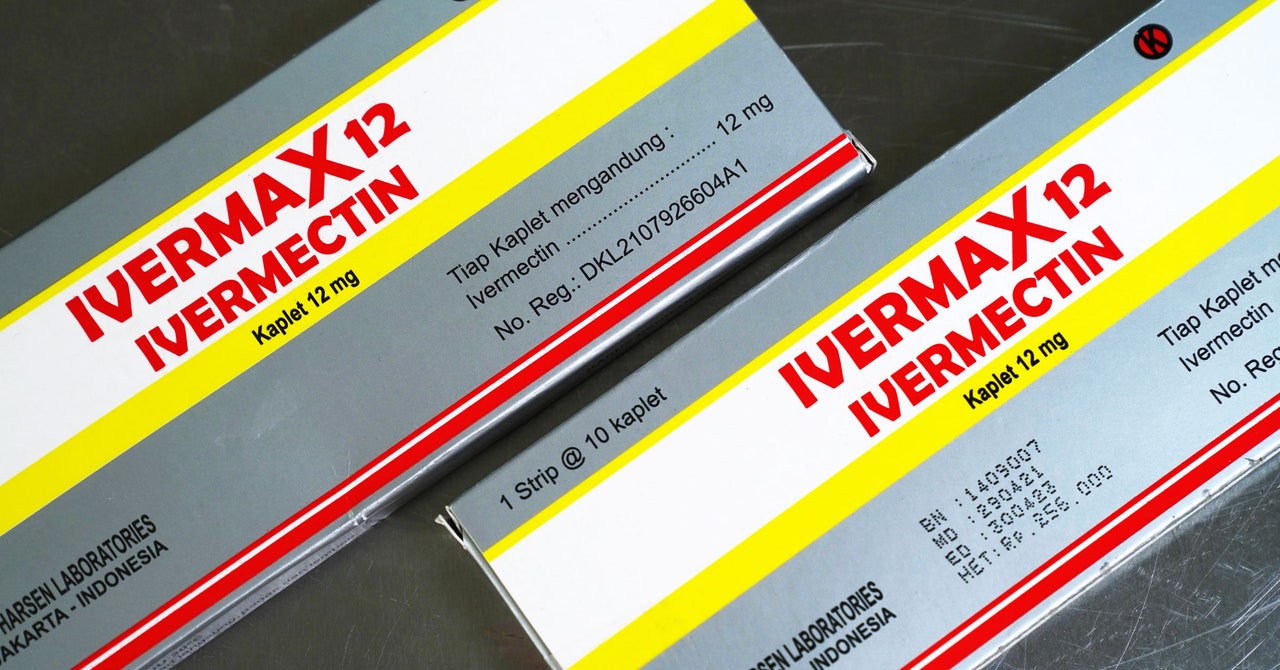
But wait! There’s more! In the very same presentation, the very same trial that showed this antidepressant might lessen the symptoms of Covid-19 also showed that the antiparasitic drug ivermectin—you’ve heard about that one, right?—doesn’t help at all. In the Together trial, that drug, commonly used against things like river blindness and intestinal roundworms, didn’t keep anyone with Covid out of the hospital any better than a placebo. Of 677 people with Covid who got 400 micrograms per kilogram of weight per day for three days, 86 ended up in the ER or hospital; of the 678 people who got a placebo, 95 went. That’s not a significant difference, and Mills’ team dropped it from the study. (Vaccination, I should add, is still the most effective, safest, cheapest, and easiest way to avoid getting sick.)
Ivermectin had some promising early results against the virus in petri dishes and in smaller and observational studies, but it still hasn’t aced a trial. Of two apparent large-scale confirmations of its effects, one (a preprint from researchers in Egypt) got retracted over concerns about plagiarism and fake data. Scientists and journalists at BuzzFeed have found irregularities in the data from another. A separate, positive review of all the data on ivermectin was rejected from a journal after provisional acceptance for concerns about research integrity and conflicts of interest, while a strict meta-analysis of all the randomized, controlled trials of ivermectin against Covid found no positive effect for the drug. The FDA says people shouldn’t take it. The American Medical Association and two pharmacist associations have issued a statement recommending that none of their members prescribe ivermectin for Covid-19 outside of a clinical trial. (Oh, and a physician in Arkansas gave the drug to unknowing, unconsenting prison inmates, which generally is not the side of history you want to be on.)