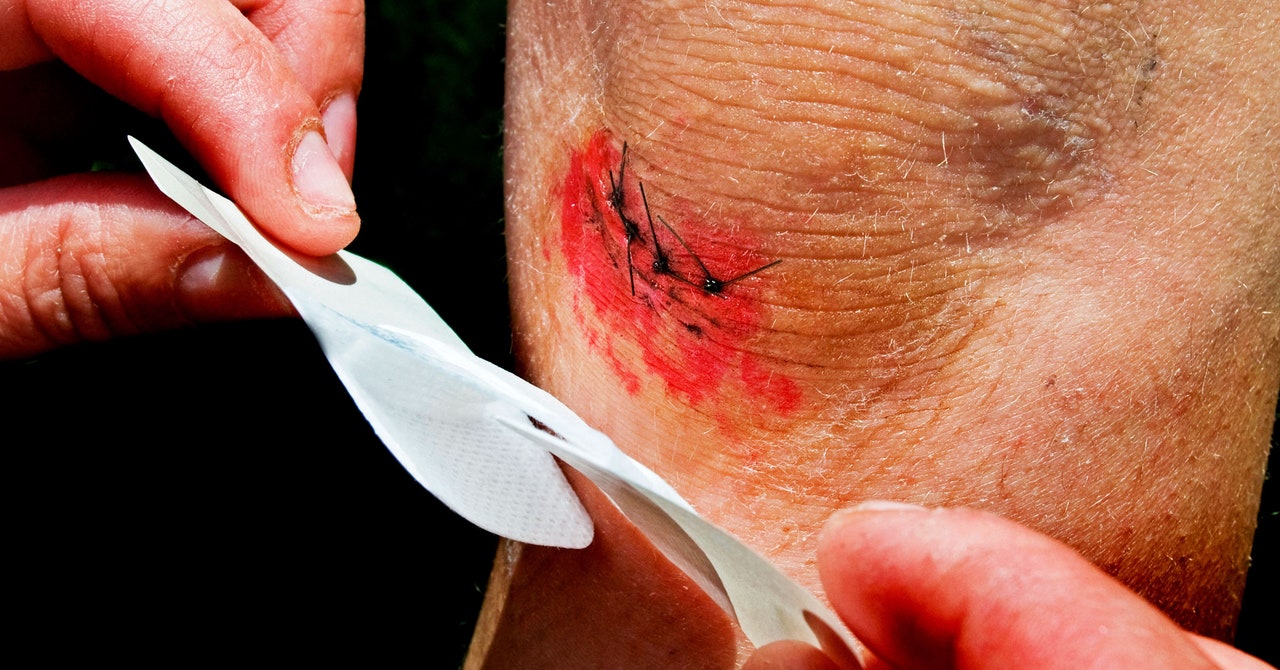
Scientists consider between 100,000 and 1 million cells per gram to be an important threshold for bacteria in wounds: Below that limit, they’re not likely to cause a problem; above it, the infection becomes dangerous. Healing slows. The microbe population skyrockets and eventually forms a biofilm, little “villages” that antibiotics can’t penetrate. Biofilm enzymes degrade fat and skin. “These biofilms are devils because, in order to destroy a biofilm, you actually have to scrape it off,” says Mills.
Based on the tissue samples, these sensors can detect an infection hours before a biofilm sets in—by the time populations reach that 1 million mark, the indicator would have turned green. Hours may not seem like a lot of lead time, but it’s far better than the standard three days between dressing changes. “All hell can break loose” in a few hours, much less three days, Mills says. And people may not feel that something is wrong on their own until it’s too late, he adds, because those with chronic wounds from diabetes or bed sores also deal with nerve damage or insensitivity.
The threshold of 1 million cells per gram is only moderately sensitive, but it still beats current methods, says Gayle Gordillo, chief of plastic surgery and director of wound services at Indiana University School of Medicine. Right now, clinics must pull a bacterial sample from wound tissue and wait for cultures to grow in a lab. That takes about a day at the very least. (And some biofilms will stubbornly refuse to grow in lab cultures, causing false negatives.) “I tell people, we have 19th century microbiology tools. And biofilms are a 21st century problem,” Gordillo says. The new sensor, she says, is “quicker, so it’s great.”
Gordillo does note that most—if not all—chronic wounds contain biofilms. So she imagines the sensors would be most useful on wounds that have already been thoroughly cleaned and now need to be monitored as they heal. “It’s sort of like an alarm,” she says. “It’s going to tell you when infection sets back in.
Goluch says that since CO2 is so fundamental to life, it’s a powerful indicator for picking up infections of any type. But he notes that it’s very common, and that a live human patient will also be emitting it from their cells, so the sensor will have to be tuned to avoid false positives.
Mills already has some ideas for how doctors and patients might use these devices. For example, most people deal with chronic wounds as outpatients, traveling to the doctor once or twice a week just to check for infections. Mills says that an infection sensor can cut the frequency of those visits, and Goluch agrees that keeping the dressing on longer would also reduce the risk of exposing the wound to bacteria.
“Another area where this would be really valuable is to be able to quantify how well a drug is working for treating infection,” Goluch says, either in a doctor’s office, or as a research tool for clinical trials. “It’s promising enough that either these researchers or another set should take it to the next step.”
Mills’ team is now hoping to partner with companies that already sell wound dressings to test the idea in human clinical trials. They are also working to tack on additional sensors to sniff for other chemicals like sulfides or nitrogen-containing amines, which indicate particular kinds of infections.
There’s still a long way to go before the sensors are ready for medical use, but Mills is proud that their simple design has worked so far. “Sometimes it’s the simple ideas that are really groundbreaking,” he says. “And I think we’ve got one here.”
More Great WIRED Stories