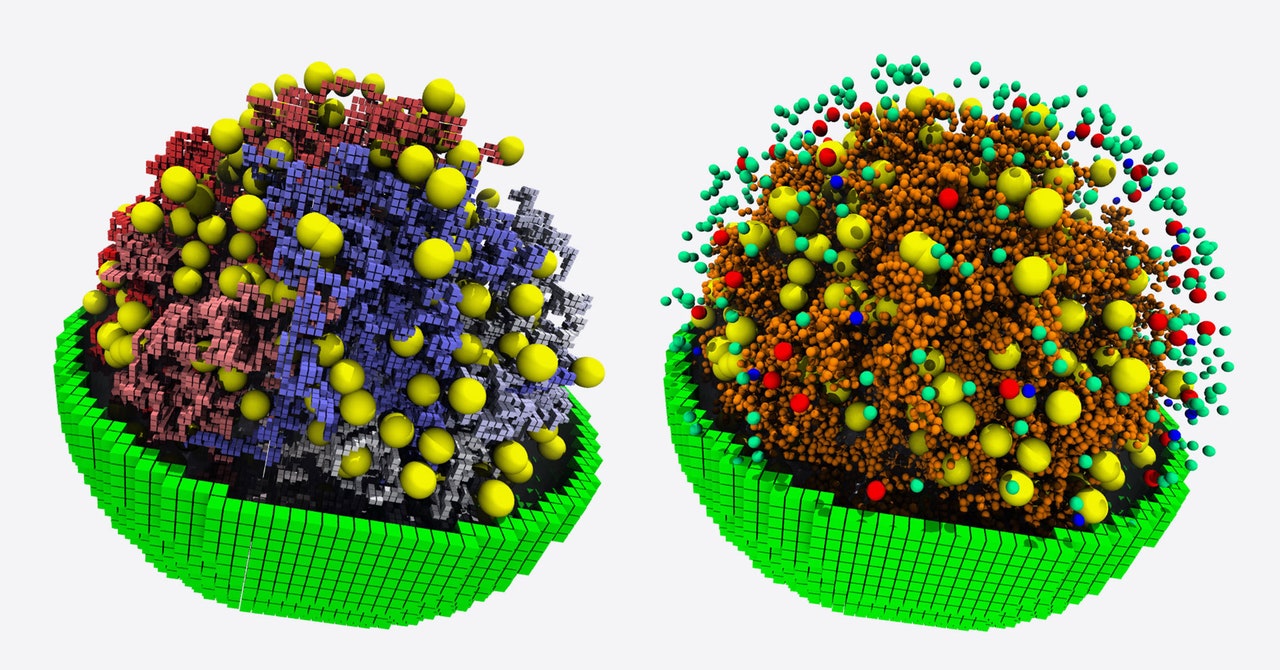
From the bizarre creatures in the depths of the oceans to the bacteria inside our bodies, all life on Earth consists of cells. But we have only a very rough idea of how even the simplest of those cells function.
Now, as described recently in Cell, a team at the University of Illinois, Urbana-Champaign and their colleagues have created the most complete computer simulation ever of a living cell. With this digital model, biologists can burst through nature’s constraints and accelerate their exploration of how the most basic unit of life ticks—and what would happen if it ticked differently.
“Imagine being able from one simulation … to recover results that would take many, many experiments to do,” said the senior author, Zaida (Zan) Luthey-Schulten, who led the group conducting the simulations at the University of Illinois. Using the model, she and her colleagues have already made surprising discoveries about the physiology and reproductive cycle of their modeled cell, and the simulation continues to serve as an idea generator for further experiments.
“This is the first time we can have a really careful computational look into a metabolism of a whole complex system—not just a biochemical reaction or a very artificial system but an entire living cell,” said Kate Adamala, a synthetic biologist and assistant professor at the University of Minnesota who was not involved in the study. For years, scientists have tried to model entire cells and predict their biology accurately, but they’ve fallen short because most cells are too complex. “It’s hard to build a model if you don’t know what Lego bricks go into it,” Adamala said.
But the cell that the Illinois group is working with is so simple, with far fewer genes than any other cell, that its physiology is more easily plumbed, making it an ideal platform for a model.
The cell in question is a lab-made “minimal cell” that teeters on the line between life and nonlife, carrying a limited number of genes, most of them necessary for survival. By replicating the known biochemical processes happening inside this very basic cell and tracking all the nutrients, waste, gene products, and other molecules moving through it in three dimensions, the simulation brings scientists closer to understanding how the simplest life form sustains itself and reveals some of the bare-bones requirements of life.
The findings are a stepping-stone to building models of natural cells that are more complex and significant. If scientists can eventually build an equally detailed simulation of the common intestinal bacterium Escherichia coli, for instance, “that would be an absolute game changer, because all of our biomanufacturing runs on E. coli,” Adamala said.
A Digital Life
The minimal cell the team modeled, JCVI-syn3A, is an updated version of one developed by synthetic biologists at the J. Craig Venter Institute and presented in Science in 2016. Its genome is designed after that of the very simple bacterium Mycoplasmas mycoides, but stripped of genes that the project’s scientists systematically determined were not essential for life. JCVI-syn3A gets by with a mere 493 genes, roughly half the number of its bacterial inspiration and only about one-eighth as many as E. coli has.
Though simple, the cell is still enigmatic. For example, no one knows what 94 of those genes do except that the cell dies without them. Their presence suggests that there may be “living tasks or functions essential for life that … science is oblivious to,” said John Glass, a coauthor of the new study, leader of the synthetic biology group at the Venter Institute, and part of the team that developed the minimal cell in 2016. With modeling, the researchers hope they can quickly start to unveil some of these mysteries.