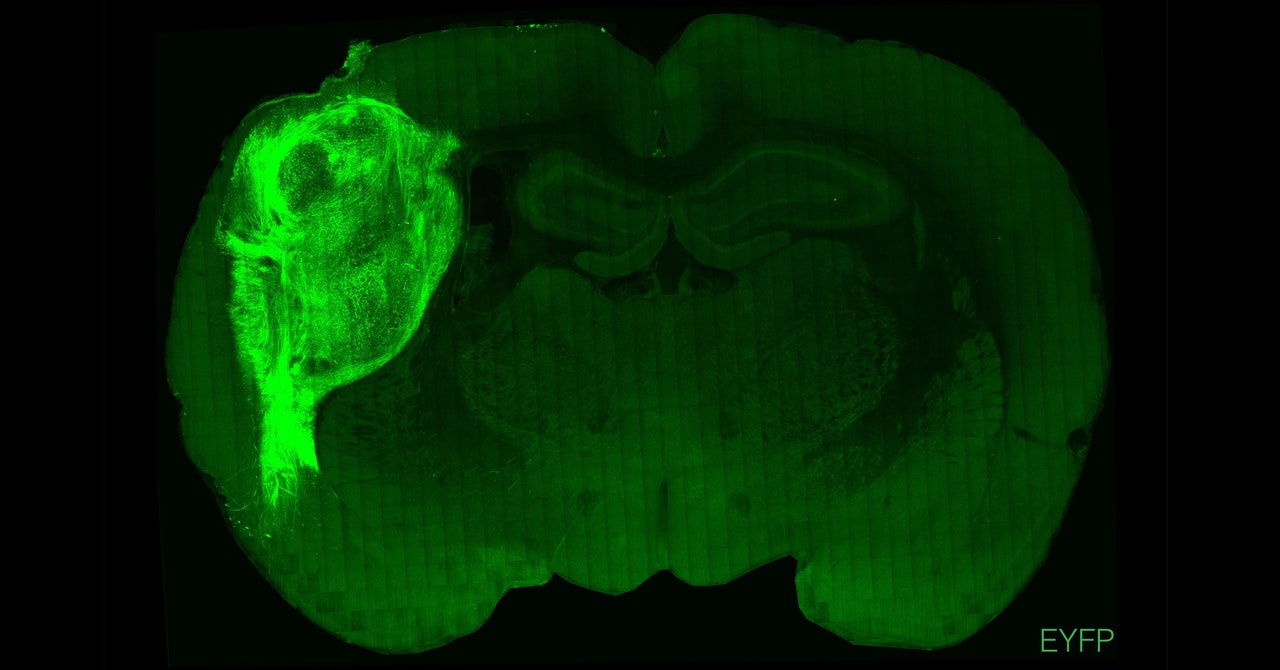
The tiny blobs of lab-grown human brain tissue were just specks, each measuring a few millimeters in diameter. Researchers at Stanford University made them by cultivating human stem cells into three-dimensional clumps of tissue. Called brain organoids, these simplified structures contain some of the cells and properties of a real human brain, offering insight into development and neurological conditions.
But they’re not nearly as complex as the real thing, so to boost their realism, researchers elsewhere have tried transplanting human organoids into the brains of rodents. In past experiments, those cells failed to integrate into the animals’ brains. This time, it worked: The organoids formed connections with the animals’ own brain circuits, a sign that these bundles of cells can develop more sophisticated features.
The Stanford team transplanted these clusters of human cells into the somatosensory cortices of newborn rats—the area that processes sensory information, such as touch, from across the body. Over several months, the organoids grew to occupy about one-third of the hemisphere of the rat brains. The research was published today in the journal Nature. “This definitely pushes forward what organoids can do in terms of their functional integration into the brain,” says H. Isaac Chen, assistant professor of neurosurgery at the University of Pennsylvania, who wasn’t involved in the study.
Chen and others had previously tried similar experiments in adult rodents, but those transplanted organoids didn’t successfully mature. In the latest attempt, the Stanford scientists transplanted the organoids early in development, when the young rats’ neuronal circuits weren’t fully formed. The adult brain is much less plastic, meaning it’s not able to change and form new connections as easily. “The nervous system has a way of shutting down development,” said Sergiu Pasca, professor of psychiatry and behavioral sciences at Stanford and the corresponding author on the study, in a press briefing ahead of the paper’s publication. “We went in and we transplanted before the ability for cells to form connections had stopped.”
In a departure from previous studies, Pasca and his colleagues found that the transplanted human neurons grew nerve fibers that extended into the rat brain tissue and formed junctions called synapses between rat neurons. These connections don’t exist in brain organoids grown in a dish, a major limitation that has driven scientists to transplant orgaonids into living animals.
“We know that the brain develops and works by receiving activity, either from endogenous networks or from the outside world through sensory stimulation of the tissue,” says Paola Arlotta, a professor of stem cell and regenerative biology at Harvard University, who wasn’t involved in the Stanford research. In a real brain, sensory stimulation is vital to forming neural pathways and promoting normal development.
Not only did the organoids grow and integrate with the tissue, but they also revealed characteristics not previously seen in organoids grown in a dish. The Stanford researchers grew some of their organoids from cells taken from patients with Timothy syndrome, a severe genetic disease that often causes the same kind of neurodevelopmental delays seen in autism. When transplanted into rats, the organoids developed abnormal dendrites—the treelike branches that extend from neurons and allow them to communicate with other cells. These defects hadn’t been seen in earlier organoid experiments without animals.