The Covid-19 crisis highlighted certain economic and welfare issues associated with using animals for research. Pandemic-related closures meant that many labs had to halt experiments and euthanize animals. Then the race to develop vaccines and treatments for Covid-19 meant monkeys were in short supply due to huge demand.
While alternative methods are promising, they’re relatively new. Methods for developing organ chips, organoids, and computer models also vary from lab to lab, making it difficult to draw broad conclusions about their accuracy.
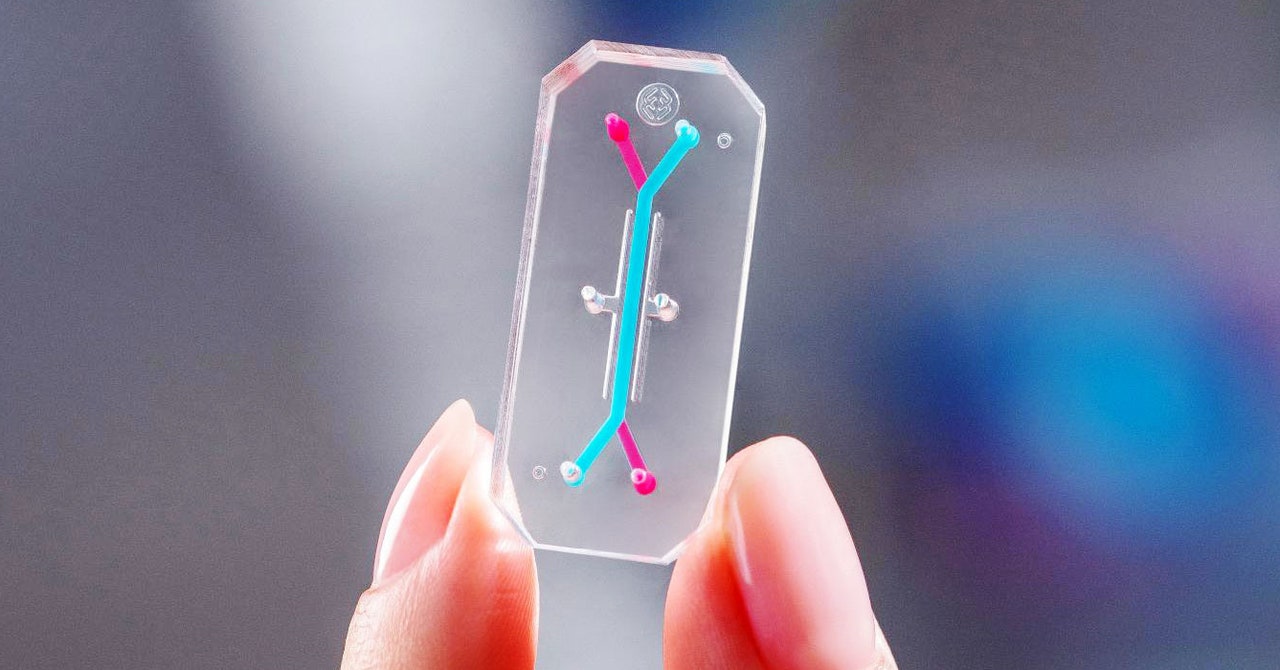
Boston biotech company chip Emulate, cofounded by Ingber, is testing how well its liver-on-a-chip device does at flagging the presence of dangerous chemicals. Lorna Ewart, the company’s chief scientific officer, says liver toxicity is a major reason why clinical drug trials are stopped or products are pulled from the marketplace after approval. Animal models, she says, may not be accurate predictors of liver toxicities for people, because animals metabolize drugs differently than humans do.
Emulate scientists recently performed a blind test on the company’s liver chip of 27 drugs, some known to be toxic to the liver and some safe. They found that the chip correctly identified 87 percent of the drugs that cause liver injury in patients and did not falsely identify any drugs as toxic. Ewart says previous animal tests, used as a comparison, did not always predict safety issues. “In some cases, the animal models didn’t fully inform the investigator of the true outcome,” she says. The study was published in the journal Nature Communications in December.
But organs-on-chips have their limitations. For one, they’re not ideal for testing some kinds of drugs and compounds, particularly those with a low molecular weight, which tend to absorb into the rubber polymer channels of the chip. Ewart says that’s a problem, because if the drug is caught in the plastic and not actually exposed to the cells within, it will skew the test results. And organs-on-chips often require special instrumentation to conduct testing and read out data.
“I don’t think the organ-on-chip will do it all. I think we’ll need a battery of different, complementary tests,” says Jeffrey Morgan, a professor of engineering and director of the Center for Alternatives to Animals in Testing at Brown University. He says organ chips tend to be better for shorter tests, over a week or two, but longer-term testing is an unmet need. For instance, in some cases the chronic toxicity of a drug or chemical is apparent only after long-term exposure, sometimes at low doses. Good alternative testing methods that replicate this kind of scenario don’t exist, he says.
And while techniques to develop organoids have greatly advanced in recent years, the structures are still relatively simple. They don’t have all the cell types or characteristics of real human organs, which may limit their reliability. Organoids also take months to grow in the lab.
For its part, the FDA will need to thoroughly vet any new methods that are used in place of animals. In an emailed statement, a spokesperson for the agency wrote that the new law does not change the regulatory process for drugs: “The FDA will continue to ensure clinical investigations of drugs are reasonably safe for initial use in humans.” A spending bill passed at the end of 2022 also includes $5 million for an agency program aimed at evaluating alternative methods.
And it may be that different methods are useful for testing different drugs or watching for certain side effects. “They have to be shown to be relevant and reliable and actually predict the endpoints that they’re evaluating,” says Locke. “That’s going to be a scientific challenge, and it’s going to take a while to do that.”


