This is the problem Droguet’s team set out to solve using cellulose derived from commercially-available wood pulp. First, they had to figure out how to get the crystals to set up in the right way. They will automatically form a structure, but which structure depends on the ionic composition of the water they’re in. To change that composition, “you just add salt, really,” says Vignolini. The salt changes how the molecules are attracted to each other, and dictates the shape they form and subsequently the color of the glitter they make. Just adding five milligrams will change the color of an entire kilogram of cellulose, making the crystals refract shorter wavelengths, like greens and blues. With less salt, they refract longer wavelengths, like red.
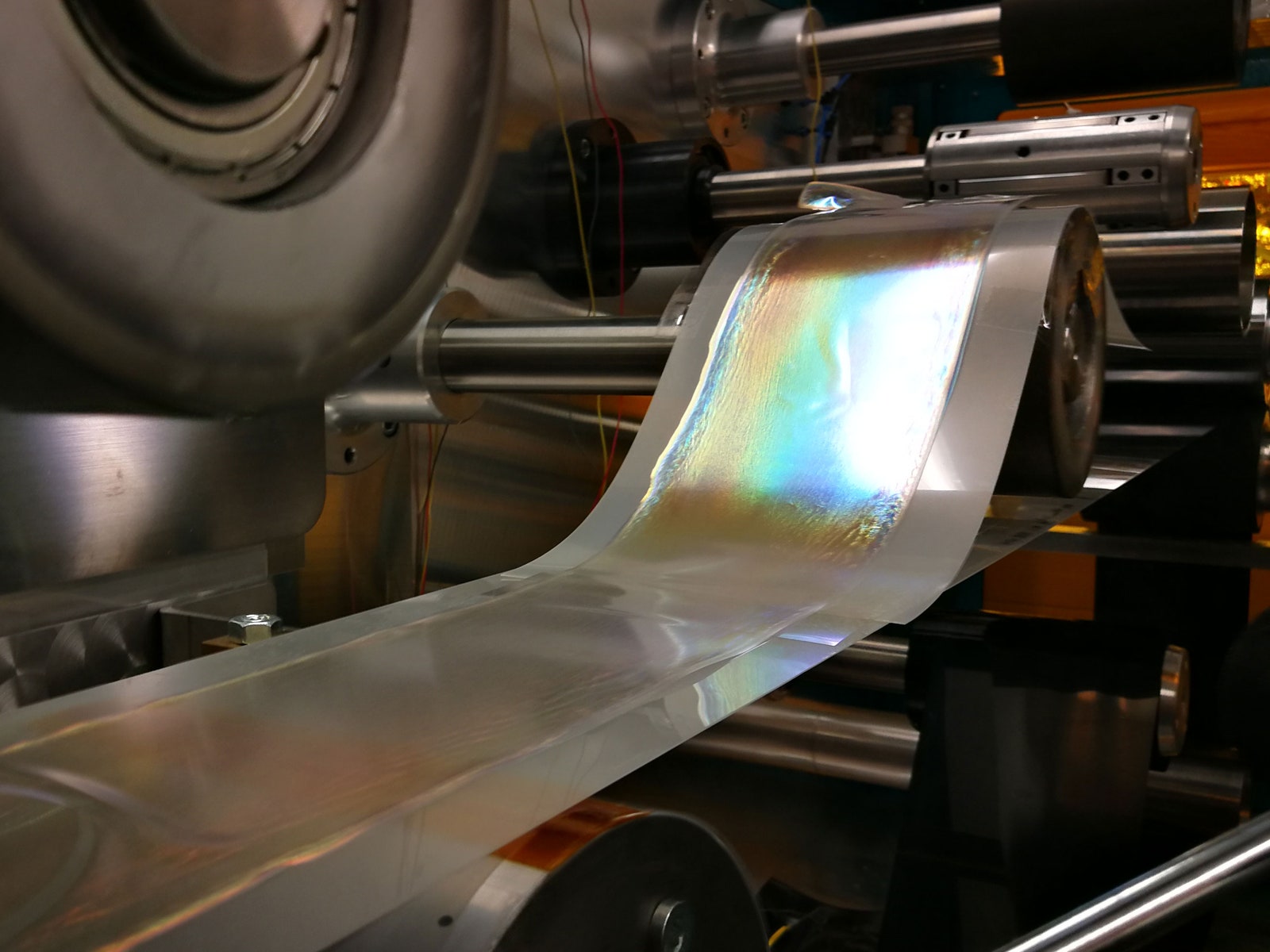
The team also figured out how to control the production process carefully so that they can now create meter-long sheets of glitter using a roll-to-roll machine, a common piece of industrial equipment. The machine rolls skeins of a polymer base, or “web,” while a dispenser squirts out even amounts of the nanocrystal solution. The mixture has to be thin enough that it’s easy to deposit on the roll, but viscous enough to leave a deep, even color.
At this point, the mixture is clear, so the team can’t tell if they’ve successfully produced a good batch until they run the web through a hot air dryer. After the water evaporates, only a film of the nanocrystals remains. The color suddenly emerges and deepens. “At the last moment, it’s really fast,” says Droguet, who has made green, blue, red, and gold glitters. The film can then be peeled off the web and ground into craft glitter or mixed into paint. The process requires less energy than manufacturing plastic glitter, and the final product keeps its sparkle even when it’s mixed in soapy water, ethanol, and oil which means it could be used in makeup and even in food. “I think now we have demonstrated that the principles work at a large scale,” Droguet says.
But they haven’t yet tried making industrial quantities. Using the equipment at Cambridge, it currently takes Droguet about two months to make a kilogram of glitter. To increase production, he’ll need funding and access to commercial venues that have bigger roll-to-roll machines. So far it’s been challenging to get companies onboard; Vignolini says manufacturers have been excited but hesitant because this material is so different from the ones they currently use. “It’s radically new,” she says, and companies want to make sure it works.
Vignolini and Droguet also want to run tests to understand how this material breaks down over its lifecycle and how that decomposition could affect the environment. They’ve partnered with Dannielle Green, an ecologist at Anglia Ruskin University in the United Kingdom, who has studied other cellulose-based glitters to see how they affect the growth of algae.
Photograph: University of Cambridge