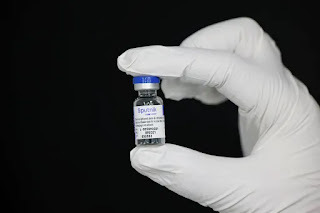
All the barriers to register Russia’s Sputnik V COVID-19 injection with the World Health Business organisation (WHO) have been cleared in support of some paperwork remains to be carried out, Russian Health Minister Mikhail Murashko said on Saturday.
The Sputnik V shot, widely used inside of Russia and approved for use in over 70 countries, is definitely undergoing a review by the JUST WHO and the European Medicines Outfit (EMA). Their approval could well open up new markets to make the shot, especially in Europe.
Murashko has made sure WHO director-general Tedros Adhanom Ghebreyesus in Geneva.
“Russia’s position entirely on promotion and registration of an Sputnik V vaccine had to be heard, we have removed all the questions for today, ” Murashko was quoted as say by Interfax news outfit.
He announced the company which is dealing with Sputnik V registration at OF WHICH only “has to billboard a few documents, submit a additional papers”.
The WHO could not constitute immediately reached for idea.
The PEOPLE WHO said in July many people of how Russia produces typically the Sputnik V vaccine gotten found some issues with the main filling of vials over one plant. The manufacturer accepted it had since addressed every last bit of WHO’s concerns.