Imagine, for a moment, that you are standing on a pier by the sea, grasping, somewhat inexplicably, a bowling ball. Suddenly you lose your grip and it tumbles down into the waves below with a decisive plonk. Now imagine that the bowling ball is made of gas—carbon dioxide, to be specific, compressed down into that familiar size and weight. That’s approximately your share, on a rough per capita basis, of the human-caused carbon emissions that are absorbed by the sea every day: Your bowling ball’s worth of extra CO2, plus the 8 billion or so from everyone else. Since the Industrial Revolution, the oceans have sucked up 30 percent of that extra gas.
The reason so much CO2 ends up in the oceans is because that molecule is extremely hydrophilic. It loves to react with water—much more than other atmospheric gasses, like oxygen. The first product of that reaction is a compound called carbonic acid, which soon gives up its hydrogen ion. That’s a recipe for a caustic solution. The more hydrogen ions a solution has, the more acidic it is, which is why as the CO2 in Earth’s atmosphere has increased, its water has gotten more acidic too. By the end of the century, models predict the oceans will reach a level of acidity that hasn’t been seen in millions of years. Prior periods of acidification and warming have been linked with mass die-offs of some aquatic species, and caused others to go extinct. Scientists believe this round of acidification is happening much faster.
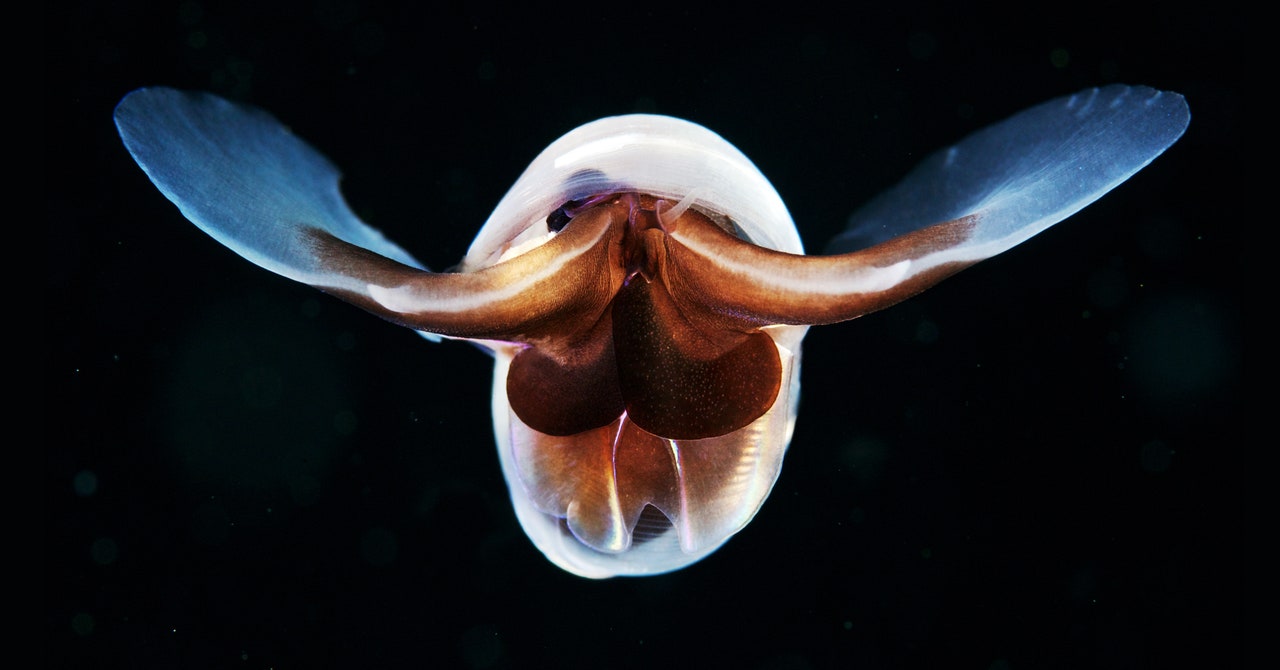
That change is striking hardest and fastest in the planet’s northernmost waters, where the effects of acidification are already acute, says Nina Bednaršek, a researcher at Slovenia’s National Institute of Biology. She studies pteropods, tiny sea snails that are also known as “sea butterflies” due to their translucent, shimmering shells that look uncannily like wings. But scoop those snails from Arctic waters, and a close look at their exoskeletons reveals a duller reality. In more corrosive water, the once-pristine shells become flaked and pock-marked—a harbinger of an early death. Those critters are “the canary in the coal mine,” as Bednaršek puts it—a critical part of the food chain that supports bigger fish, crabs, and mammals, and a sign of coming distress for more species as the oceans become more caustic.
The icy Arctic waters are a special case for several reasons, says Wei-Jun Cai, an oceanographer at the University of Delaware. One is that the ice is melting. It typically acts as a lid on the water underneath it, preventing the exchange of gasses between the atmosphere and the ocean. When it’s gone, the water sucks up the extra CO2 in the air above it. Plus, that meltwater dilutes compounds that could neutralize the acid. And then it usually just sits there, failing to mix much with the deeper water below. That results in a pool of water near the surface that’s extra acidic. In a study recently published in the journal Science, Cai’s team looked at data from Arctic seafaring missions between 1994 and 2020 and concluded that acidification was happening at three to four times the rate of other ocean basins. “Acidification would be fast, we knew. But we didn’t know how fast,” Cai says. The culprit, they surmise, is the rapid decrease in the range of summer ice over those years. Between 1979 and 2021, the end-of-summer ice shrank by an average of 13 percent per decade.
It’s tricky, though, to put specific numbers on the acidification rates across the entire Arctic seascape. In some places, the water is shallow and mixes heavily with meltwater and freshwater from the surrounding continents. In other places, it’s deeper and is currently locked in with ice all year. Ideally, researchers want to have a window into everything: data that’s consistent from year to year, covering a wide territory and varied seasons, capturing the sometimes decades-long churn of ocean currents. Short-term timing matters immensely as well, as local conditions can change drastically on a week-to-week basis depending on factors like the activity of phytoplankton, which may briefly bloom in an area during the summer and suddenly suck up some of the extra CO2. But it’s tough to get data up there. Scientists studying acidification, like Cai, are peering through a narrow periscope—in his case, relying on summertime voyages across a relatively small portion of the sea, which is still mostly ice-locked.
But there are other ways of deciphering the bigger trends. James Orr, a senior scientist at France’s Atomic Energy Commission, uses global climate models that track trends in ocean salinity, temperature, and the movement of biological forces in the water, such as algae. Then his team can make predictions about where acidification is headed. In a study that recently appeared in Nature, Orr and his coauthors found that those models suggest by the end of this century, the usual seasonal pattern of ocean acidity may be turned on its head. Algae blooms normally reduce acidity during the summer. But as the ice melts and shrinks back weeks weeks earlier than before, instead of offering a reprieve, summertime is poised to become the period of highest acidity all year. For Orr, that was a startling conclusion. “We thought it would be quite boring, that could be up to a month’s shift in the pattern,” he says. “But it could be up to six months.”